Abstract
Background/Introduction: 4-factor prothrombin complex concentrate (PCC) is currently approved in the United States for the urgent reversal of warfarin-induced coagulopathy in adults with acute major bleeding. However, the way in which 4-factor PCC is used in a real-world setting and the outcomes associated with off-label use is less clear. The objective of this study is to identify the indications that lead to 4-factor PCC use and the associated outcomes at a single university hospital.
Methods: This was a single center retrospective cohort study of patients who received 4-factor PCC for any indication between July 2015 and April 2017 at Oregon Health & Science University. Patients were followed from index date until hospital discharge or in-hospital death. A given dose was considered "on-label" if it was given specifically for major bleeding secondary to warfarin-induced coagulopathy, whereas doses given for any other reason were considered "off-label". Patients were also categorized based on the specific indication for receiving 4-factor PCC, which included intracranial hemorrhage, trauma-related bleeding, non-trauma-related bleeding, reversal of coagulopathy prior to an urgent procedure in non-bleeding patients, and intra- or post-operative bleeding. Also documented were baseline INR/PT/PTT and which form of anticoagulation, if any, a patient was on at baseline. Outcomes included duration of time between dose and INR normalization (defined as INR <1.5), duration of time between dose and ordering of follow-up coagulation parameters, duration of time between dose and hemostasis (determined based on vital signs, hemoglobin measurements, and/or imaging assessments), incidence of thromboembolic complications, and survival to hospital discharge.
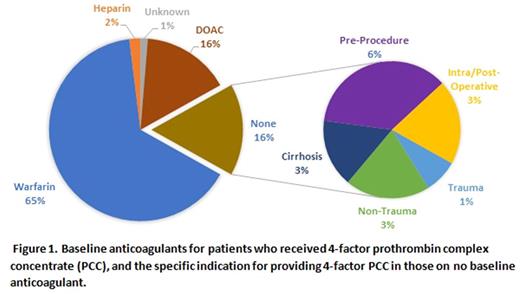
Results: In total,154 patients received 4-factor PCC during the study period accounting for 165 total doses. Ninety doses (54.5%) were considered on-label, while 75 doses (45.5%) were considered off-label. Of these off-label doses, 61 doses (81.3%) were considered off-label as they were given to patients not on warfarin therapy at baseline, while 14 doses (18.7%) were considered off-label due to lack of major bleeding. Intracranial hemorrhage was the most common indication (91 doses, 55.2%), followed by non-trauma-related bleeding (26 doses, 15.8%), pre-procedural reversal of coagulopathy without bleeding (21 doses, 12.7%), intra- and post-operative bleeding (13 doses, 7.9%), trauma-related bleeding (12 doses, 7.2%), and reversal of coagulopathy without bleeding in those not undergoing a procedure (2 doses, 1.2%). One-hundred patients (64.9%) were on warfarin at baseline, while the remainder were on direct oral anticoagulants (24 patients, 15.6%), heparin (3 patients, 1.9%), or no anticoagulation (25 patients, 16.2%), as depicted in Figure 1.
Comparing on-label versus off-label use of PCC, there was no difference in time to INR normalization (p=0.24) or time to hemostasis (p=0.25). There was significantly shorter duration of time to ordering follow-up coagulation parameters in on-label versus off-label use (2.52 hours vs 3.97 hours, p=0.04). Comparing on-label versus off-label use, there was no difference in incidence of thromboembolic complications (8.9% vs 5.9%, p=0.76) or survival to hospital discharge (81.4% vs 67.6%, p=0.06).
Conclusion: Despite being approved only for major bleeding related to vitamin K-antagonist use, 4-factor PCC is often administered in a wide variety of other settings in patients without major bleeding and those not on warfarin therapies. With the exception of duration of time to ordering follow-up coagulation parameters, outcomes in this single-center analysis comparing on-label versus off-label use were generally equivalent. Given the frequent use of 4-factor PCC outside of its currently approved indication, larger scale prospective trials studying specific indications are needed to validate its use in these settings.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal